Aluminum:
Aluminum capacitors are the workhorses of big capacitors, they are small and cheap for their capacity and can be found in sizes
from <1 uF to over 1 farad. They are commonly available to 450 volts working voltage, with a few to at least 600 volts, much
higher than other types of electrolytic capacitors. Aluminum electrolytic capacitors use a layer of aluminum oxide (Al2O3 with a
K of about 8.5) grown on aluminum foil. The aluminum foil forms one electrode while the other is a non-aqueous electrolyte
(hence the name) in a thin paper separator, and another foil layer for the cathode. The separator holds the electrolyte and
keeps the cathode from rubbing on the dielectric and damaging it. The classic electrolyte formulas date back to the 1930s and
were usually a glycol or amine (a solvent), in which a conductive salt (a solute) is dissolved, plus 1-2% water. The water raises
the electrolyte conductance and promotes healing of the aluminum oxide. Many variations on this have been used over the
years, although ethylene glycol is still much used. A chemical called gamma-butyrolactone is commonly used in high-
temperature electrolytics. The various solutes include benzoic acid, salicylic acid and adipic acid. A patent search will find any
number of exotic electrolytes, but who knows how many of these are actually in production. Given the variations that aluminum
electrolytics are found in however, there may be many propriety electrolytes in use. The electrolyte is specialized enough that
many small pacific-rim cap makers buy their electrolyte (often from Japan) rather than make it themselves. Some chemicals in
the electrolytes may be toxic to some extent. To make a non-polarized capacitor, the other foil electrode is also oxide coated.
The reason for the electrolyte is that the surface of the oxide-coated foil electrode is etched to greatly increase the surface area.
The ratio of capacitance with etched foil vs unetched foil is called "foil gain", and is typically 25-100 for modern aluminum
electrolytics, depending on voltage. The other foil electrode can't make intimate contact with the dielectric by itself so the
electrolyte is used as an intermediary conductor. Etching the foil is a science in its own right. High-voltage electrolytics require
a different surface than low-voltage due to the different thickness in oxide coating required. There has been a slow-but-steady
improvement in etching that has improved volumetric efficiency over the years, roughly doubling over the last decade. As
manufacturers phase in the improved foil, they will obsolete the capacitor series that use the old foil and replace them with
smaller parts that use the new foil. However, the reduced-volume parts tend to have slightly higher ESRs than the equivalent
older higher-volume parts.
Almost all aluminum electrolytic capacitors are sealed with a crimped rubber plug (or "bung"). The common rubbers are
variations on ethylene propylene rubber (EPR), isobutylene-isoprene copolymer (butyl rubber), styrene butadiene rubber
(SBR), natural rubber (polyisoprene, NR), and ethylene propylene diene monomer (EPDM). EPDM is becoming the more
popular, it has superior resistance to almost everything. Fillers are usually added to improve mechanical properties. Sealing the
capacitor has to be done just right for longest life. Too loose a seal can allow the capacitor to dry out. However, aluminum
electrolytics always generate some small amount of hydrogen and oxygen that must be vented off by diffusion through the bung
to prevent pressure buildup.
Common cleaning solvents can be a problem for the plug. Alcohol is generally OK but chlorinated solvents will cause corrosion
and none of the rubber formulations will keep solvents out. Some electrolytics are made solvent-proof with epoxy.
Aluminum electrolytics have their problems, however; noise, high leakage, high temperature drift, high dielectric absorption, high
inductance, high almost everything bad. Low temperature is a problem for most aluminum capacitors. For most types,
capacitance falls off rapidly below room temperature while dissipation factor can be ten times higher at -25C than at 25C.
Most limitations can be traced to the electrolyte. At high temperature, the water can be lost to evaporation, and the capacitor
(especially the small sizes) may leak outright. At low temperatures, the conductance of the salts declines, raising the ESR, and
the increase in the electrolyte´s surface tension can cause reduced contact with the dielectric. The conductance of electrolytes
generally has a very high temperature coefficient, +2%/C is typical, depending on size. The electrolyte is implicated in various
reliability issues as well.
Because of aluminum capacitor's problems, other capacitor types compete for aluminum's turf in some applications. In small
sizes, tantalum capacitors are available, but at a higher cost. Ceramic capacitors are available into the 100s of uF and film
capacitors are now available >1000 uF.
Aluminum capacitors are at their best doing simple jobs like line-frequency power supply filtering. If you want to use them for
more demanding applications, like switcher power supply filtering (low ESR), audio DC blocking (low-noise), or in high
temperature environments (long life), you must use care in selecting one the of special-purpose aluminum capacitors. There are
many to choose from, however; apparently the basic technology gives capacitor designers a lot of room to maneuver. They
include:
•Low noise for audio applications.
•Low leakage for RAM backup and even timing applications. Vendors sometimes tout these as tantalum replacements
•Low equivalent series resistance (ESR) and/or low inductance (ESL) for switcher power supply filtering or for high
ripple-current applications. There are even 4-lead types available.
•High-temperature types for improved life and reliability at elevated temperatures. Rated operating temperatures go to
130C for common capacitors, but at the cost of increased size. 150C types for automotive applications have started to
appear. These have a special construction to resist vibration. Special types (rare) are available for operation to 200C.
•Low-temperature types, as low as -65C. Very high and very low temperature rating may not be found in the same
capacitor.
•Non-polarized for speaker crossover networks and other audio applications.
•A variety of combination types such as high temperature + high reliability.
•Photo-flash capacitors to handle very high surge currents.
•Miniature sizes and special shapes (tall and skinny, short and fat).
•Axial leads, radial leads, screw terminals, tabs with holes for screws, quick-connect terminals and snap-in. DC link
aluminums will have multiple tabs attached to the foil to reduce the ESR and ESL.
•SMD versions are now widely used in consumer electronics. Most SMD aluminum capacitors are just a modified
version of the conventional aluminum can. True SMD aluminums are stating to appear however. A very few come in
molded packages that might at first glance be mistaken for SMD tantalums.
High temperature in general: I'll move this someday.
Tantalum:
Tantalum electrolytic capacitors are a step up from aluminum capacitors. They come in a number of types with different
advantages, but in general, they have smaller size, lower leakage, lower dissipation factor, lower ESR, more stable capacitance
over temperature, and good service life. Tantalum capacitors aren't made in the monster sizes that aluminum capacitors are, but
are available to several hundred µF in common voltage ratings (to about 100 volts) and to several thousand uF at low voltage
(6-10 volts).
The first tantalum capacitors were made from tantalum foil with a sulfuric acid electrolyte. Foil tantalums are made for use to at
least 300 volts, and to 125C. They are made in both industrial and military styles but their usage is probably mostly military.
Foil tantalums are now rare however, and seem be out of production.
The foil anode is a poor use of what is an expensive metal, and not in good supply. Most modern tantalum capacitors are what
are called "slug" capacitors. Tantalum powder is sintered into a porous yet strong slug, with a tantalum wire, which forms the
anode of the capacitor. The many small particles produce a very high surface area. A variety of particle shapes are used,
depending on the operating voltage, and making them is a science in itself. A layer of tantalum pentoxide (Ta2O5 with a K of
about 25) is grown over the particles for the dielectric. In one version of the capacitor, the electrolyte is gelled sulfuric acid.
This is called a "wet-slug" and, as you can imagine, it must be well sealed. Wet-slug tantalums are the capacitor of choice for
some applications, especially at very high temperatures (to 200C for some, often with derating). They are available for up to
900 volts operation. Construction details vary.
The vast majority of solid tantalums are the "dry-slug" or "solid tantalum" capacitors. A layer of manganese dioxide (MnO2) is
layered over the pentoxide followed by a layer of colloidal graphite and a layer of silver paint. There are minor variations on this.
With no liquid involved, they can be sealed with just an epoxy dip, although the better ones may have a molded body. The
pentoxide layer is prone to defects, and the key to the solid tantalum capacitor's reliability is that the MnO2 provides self
healing. If a flaw in the pentoxide layer develops, the leakage will cause localized heating in the MnO2 and convert it to
Mn2O3 , a much less conductive oxide, sealing off the flaw. This mechanism is not perfect, however, and failures due to
dielectric flaws have been a traditional problem for solid tantalums. If the temperature of the pentoxide gets high enough, about
500C, the pentoxide converts from its nonconducting amorphous form to its conducting crystalline form and the capacitor goes
up in flame. Solid tantalums aren't usually made with high working voltages because the particle size limits the dielectric
thickness that can be grown; 50 volts is usually the upper limit. There are however, a very few solid tantalums with working
voltages as high as 125 volts. Most dry tantalums are rated to no more than 85C to 125C, but a few are rated for use to 175C
with voltage derating. Solid tantalum capacitors are commonly available in surface-mount packages, in molded bodies.
A weakness of slug tantalums is high-frequency performance. Depending on construction details, the capacitance can fall to
50% in the 100-200 kHz region, compared to 1 kHz. Lower ESR parts can be made using larger particles (although at the
expense of somewhat lower capacitance) and by various manufacturing refinements.
A rarely mentioned characteristic of solid tantalums is their rapid decline in leakage current. When both aluminum and tantalum
electrolytics are powered up, their leakage current starts high, but declines over time. For aluminums, leakage take minutes to
decline to a stable value. For tantalums, this occurs in seconds.
Even though "dry-slug" tantalums are not wet-chemistry devices, they are still polarized. Although many sources claim that dry
tantalums are very sensitive to reverse bias, they can actually to be very slow to fail when reverse biased. In fact, failure of
tantalums installed backwards may occur from seconds to more than a year after manufacture.
Because of the growing popularity of tantalum in miniature electronic equipment, tantalums (mainly SMD) have diverged into a
number special types. They include:
•High temperature, to at least 200C with voltage derating, MnO2.
•Low ESR using specially sized powders and multiple anodes.
•Miniature package sizes.
•Very low height. Standard SMD tantalums are 1.9 to >4 mm in height. Low profile are 1.2 or 1.5 mm.
•Fused, for power supply bypassing on digital boards.
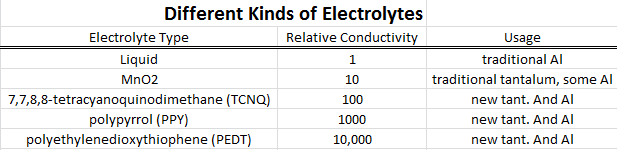
•Tantalum capacitors with the semiconducting polymer "electrolyte" TCNQ have appeared. Sanyo, for one, makes
them under the POSCAP name. See Sanyo OS-CON capacitors above. They tend to have the same advantages as
their aluminum cousins, including lower ESR and flatter temperature characteristics. Manufacturers also claim that they
can fail without bursting into flame. A new conductive polymer, PEDOT has also appeared, see above.
Companies that advertise wet-electrolyte tantalum capacitors include:
http://www.transitor.com I think all Transitor does is tantalum. They are now a Vishay company.
Mallory Gone.